The hardness of water is due to the presence of soluble bicarbonates, chlorides and sulfates of calcium and magnesium. Hard water does not give lather with soap.
Water can be classified as hard water and soft water.
- Soft water: It lathers with soap. Water which is obtained from the rains is soft water. This water is suitable for household purposes for example laundry and cleaning.
- Hard water: It is known as hard water because of the presence of salts of calcium and magnesium. Hard water does not lather with soap but instead forms a precipitate.
What is Hard Water?
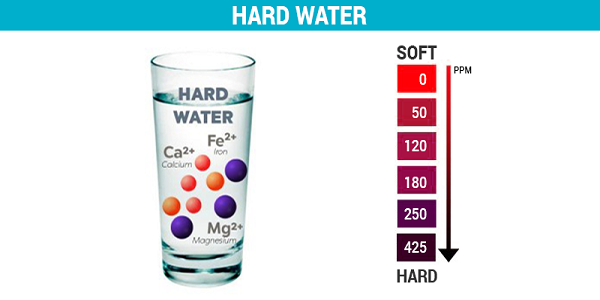
Hard water has high mineral content. It is formed when water percolates through the deposits of chalk and limestone which are made up of magnesium and calcium carbonates. It does not lather with soap, so it is not suitable for laundry purposes.
The hardness of water is harmful to the boilers as the deposition of salts occurs, which reduces the efficiency of the boiler. Hard water is safe to drink but using over a long interval of time can lead to many problems like:
- Strains in skin
- Water appliances work harder resulting in higher water bills
- Spots appear on clothes and linens
Types of Hardness of Water
The hardness of water can be classified into two types:
- Temporary Hardness
- Permanent Hardness
Temporary Hardness:
The presence of magnesium and calcium carbonates in water makes it temporarily hard. In this case, the hardness in water can be removed by boiling the water.
When we boil water the soluble salts of Mg(HCO3)2 is converted to Mg(OH)2 which is insoluble and hence gets precipitated and is removed. After filtration, the water we get is soft water.
Permanent Hardness:
When the soluble salts of magnesium and calcium are present in the form of chlorides and sulphides in water, we call it permanent hardness because this hardness cannot be removed by boiling.
We can remove this hardness by treating the water with washing soda. Insoluble carbonates are formed when washing soda reacts with the sulphide and chloride salts of magnesium and calcium and thus, hard water is converted to soft water.
Disadvantages of Hardness
- Wastage of soap
- Wastage of fuel
- Formation of scales on metallic boilers.

thank you sir