Elimination Reactions:
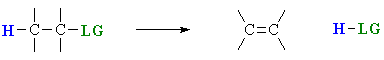
Elimination reactions are important as a method for the preparation of alkenes. The term “elimination” describes the fact that a small molecule is lost during the process. A 1,2-elimination indicates that the atoms that are lost come from adjacent C atoms.
The two most important methods are:
- Dehydration (- H2O) of alcohols, and
- Dehydrohalogenation (- HX) of alkyl halides.
There are three fundamental events in these elimination reactions:
- removal of a proton
- formation of the CC π bond
- breaking of the bond to the leaving group
Depending on the relative timing of these events, different mechanisms are possible:
- Loss of the LG to form a carbocation then removal of H+ with formation of C=C bond (two steps) : E1 reaction
- Simultaneous H+ removal, C=C bond formation and loss of the LG (one step) : E2 reaction
- Removal of H+ to form a carbanion then formation of C=C bond with loss of the LG (two steps) : E1cb reaction
In many cases the elimination reaction may proceed to alkenes that are constitutional isomers with one formed in excess of the other. This is described as regioselectivity.
E1 Mechanism:
E1 indicates a elimination, unimolecular reaction, where rate = k [R-LG].
This implies that the rate determining step of the mechanism depends on the decomposition of a single molecular species.
Overall, this pathway is a multi-step process with the following two critical steps:
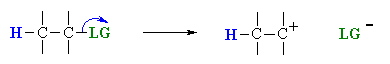
loss of the leaving group, LG, to generate a carbocation intermediate, then
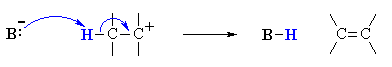
loss of a proton, H+, from the carbocation to form the π-bond.
Let’s look at how the various components of the reaction influence the reaction pathway:
R–
Reactivity order :
![]()
In an E1 reaction, the rate determining step is the loss of the leaving group to form the intermediate carbocation. The more stable the carbocation is, the easier it is to form, and the faster the E1 reaction will be. Some students fall into the trap of thinking that the system with the less stable carbocation will react fastest, but they are forgetting that it is the generation of the carbocation that is rate determining. Since carbocation intermediates are formed during an E1, there is always the possibility of rearrangements (e.g. 1,2-hydride or 1,2-alkyl shifts) to generate a more stable carbocation. This is usually indicated by a change in the position of the alkene or a change in the carbon skeleton of the product when compared to the starting material.
–LG
The only event in the rate determining step of the E1 is breaking the C-LG bond. Therefore, there is a very strong dependence on the nature of the leaving group, the better the leaving group, the faster the E1 reaction will be. In the acid catalysed reactions of alcohols, the -OH is protonated first to give an oxonium ion, providing the much better leaving group, a water molecule (see scheme below).
B
Since the base is not involved in the rate determining step, the nature of the base is unimportant in an E1 reaction. However, the more reactive the base, the more likely an E2 reaction becomes.
Selectivity:
E1 reactions usually favour the more stable alkene as the major product : i.e. more highly alkyl substituted and trans- > cis-
This E1 mechanistic pathway is most common with:
- good leaving groups
- stable carbocations
- weak bases.
A typical example is the acid catalysed dehydration of 2o or 3o alcohols.
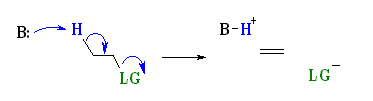
E2 Mechanism:
E2 indicates an elimination, bimolecular reaction, where rate = k [B][R–LG].
This implies that the rate determining step involves an interaction between these two species, the base B, and the organic substrate, R–LG
This pathway is a concerted process with the following characteristics:
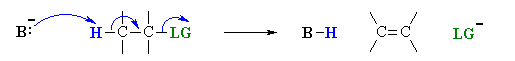
Simultaneous removal of the proton, H+, by the base, loss of the leaving group, LG, and formation of the π-bond
Let’s look at how the various components of the reaction influence the reaction pathway:
Effects of R–
Reactivity order :
![]()
In an E2 reaction, the reaction transforms 2 sp3 C atoms into sp2 C atoms. This moves the substituents further apart decreasing any steric interactions. So more highly substituted systems undergo E2 eliminations more rapidly. This is the same reactivity trend as seen in E1 reactions.
–LG
The C-LG bond is broken during the rate determining step, so the rate does depend on the nature of the leaving group.
However, if a leaving group is too good, then an E1 reaction may result.
B
Since the base is involved in the rate determining step, the nature of the base is very important in an E2 reaction.
More reactive bases will favour an E2 reaction.
Stereochemistry:
E2 reactions occur most rapidly when the H–C bond and C–LG bonds involved are co-planar, most often at 180o or antiperiplanar. This sets up the s bonds that are broken in the correct alignment to become the p bond.
Selectivity :
The outcome of E2 reactions is controlled by the stereochemical requirements described above. Where there is a choice, the more stable alkene will be the major product.
The E2 pathway is most common with:
- high concentration of a strong base
- poorer leaving groups
- R–LG that would not lead to stable carbocations (when the E1 mechanism will occur).
