Optical isomerism is basically a type of stereoisomerism. Isomers are those compounds which have the same molecular formula but different bonding arrangement among atoms.
Whereas, in stereoisomer, both molecular formula and bonding arrangement of atoms are the same. However, they have different three dimensional arrangement of atoms. It eliminates all different arrangements that are simply due to the molecule spinning in its entirety or revolving around unique bonds.
What is Optical isomerism?
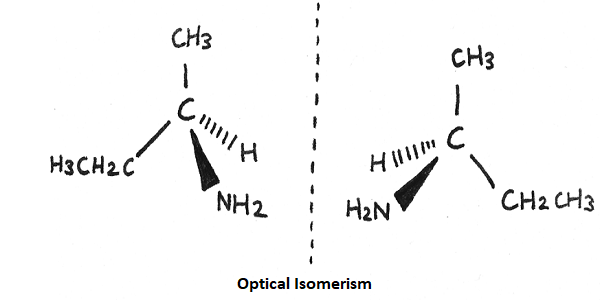
Optical isomerism is a case where the isomers display identical characteristics in terms of molecular weight as well as chemical and physical properties. However, they differ in their effect on the rotation of polarized light.
Optical isomerism occurs mainly in substances that have the same molecular and structural formula, but they cannot be superimposed on each other. In simple words, we can say that they are mirror images of each other. Alternatively, it can also be found in substances that have an asymmetric carbon atom.
Typically, optical isomerism is shown by stereoisomers which rotate the plane of polarized light. If the plane of polarized light passing through enantiomer solution rotates in the clockwise direction then the enantiomer is said to exist as (+) form and if the plane of polarized light rotates in anti-clockwise direction then the enantiomer is said to exist in (-).
For example, an enantiomer of alanine (amino acid) which rotates the plane of polarized light in clockwise and anti-clockwise direction can be written as (+) alanine and. (-) alanine respectively.
The extent of rotation of plane-polarized light by the two enantiomeric form is exactly the same but the direction of rotation is opposite. Moreover, if the two enantiomer pair are present in equal amount then the resultant mixture is called a racemic mixture. This means that 50% of the mixture exists in (+) form and the other 50% exist in (-) form.
Since racemic mixture rotates the plane of polarized light equally in the opposite direction, the net rotation is zero. Therefore racemic mixture is optically inactive.
