There is much debate concerning how wet and dry surfaces affect friction. There is no really good answer without knowing what one surface is wetted with. If, for example, a surface was wetted with oil, the coefficient of friction would decrease. The oil provides a thin film that slips easily between its laminate layers, allowing the two surfaces to slip without touching each other.
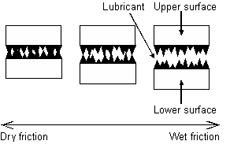
Consider, two smooth surfaces sliding over one another. If a layer of water were added between the two surfaces, the coefficient of friction would decrease. This is do to the hydrodynamic pressure that has been generated between the two surfaces pressing down on each other. The layer of water provides a mechanism on which to slip easily. The same is true if one were to add water with heavy concentrations of Na- and Cl- ions between the layers. Equal amount of the Na- and Cl- ions would adhere to each surface and the electrostatic forces between the like ions would provide a force counter to the normal, helping the two surfaces slide easily.
If the surfaces are rough, or if one surface is rough, what we think of as “wetting” would be useless. “Wetting” generally applies to water based or like liquids being applied to a surface. These liquids have no properties that make them good lubricants. They have strong molecular adhesion, there are no long organic chains holding the liquid together, and there are no active ends that bind to the surfaces of our sliding system. If the surfaces have a surface roughness greater than the atomic diameter of the liquid, the liquid does nothing to help minimize friction. In this case, the liquids are lost in the surface defects and add nothing to the system. It is even the case that these wet surfaces are harmful to sliding surfaces.
The jist of it is, if the liquid isn’t being used as a medium for hydrodynamic lubrication, hang it up. When we think about wetting a surface to minimize friction, we are thinking about adding boundary lubrication to the system. Most of the liquids we consider using to “wet” a surface though, are terribly inept at providing boundary lubrication. They have none of the physical properties necessary in a boundary lubrication. Wetting does provide a means by which to cool a system as it generates heat through friction. Heat often leads to thermal breakdown of substances. If the “wetting” substance provided a means to transport or dissipate heat then it would be useful.
