What is Water Hardness:
The simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. Hard water is high in dissolved minerals, both calcium and magnesium. You may have felt the effects of hard water, literally, the last time you washed your hands. Depending on the hardness of your water, after using soap to wash you may have felt like there was a film of residue left on your hands. In hard water, soap reacts with the calcium (which is relatively high in hard water) to form “soap scum”. When using hard water, more soap or detergent is needed to get things clean, be it your hands, hair, or your laundry.
Hard water is water that contains cations with a charge of +2, especially Ca2+ and Mg2+. These ions do not pose any health threat, but they can engage in reactions that leave insoluble mineral deposits. These deposits can make hard water unsuitable for many uses, and so a variety of means have been developed to “soften” hard water; i.e.,remove the calcium and magnesium ions.
But hard water can have some benefits, too. Humans need minerals to stay healthy, and the National Research Council (National Academy of Sciences) states that hard drinking water generally contributes a small amount toward total calcium and magnesium human dietary needs.
Measures of Water Hardness:
Hardness is caused by compounds of calcium and magnesium, and by a variety of other metals. General guidelines for classification of waters are: 0 to 60 mg/L (milligrams per liter) as calcium carbonate is classified as soft; 61 to 120 mg/L as moderately hard; 121 to 180 mg/L as hard; and more than 180 mg/L as very hard.
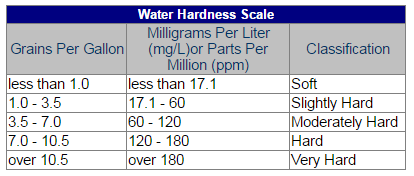
Often, when you purchase a new dishwasher or washing machine, the manufacturer has recommended settings that depend on the hardness of the water. The table below provides the historical range of “hardness” of water in the Fairfax Water system.

Problems with Hard Water:
Mineral deposits are formed by ionic reactions resulting in the formation of an insoluble precipitate. For example, when hard water is heated, Ca2+ ions react with bicarbonate (HCO3–) ions to form insoluble calcium carbonate (CaCO3), as shown in Equation 1.
![]()
This precipitate, known as scale, coats the vessels in which the water is heated, producing the mineral deposits on your cooking dishes. In small quantities, these deposits are not harmful, but they may be frustrating to try to clean. As these deposits build up, however, they reduce the efficiency of heat transfer, so food may not cook as evenly or quickly in pans with large scale deposits. More serious is the situation in which industrial-sized water boilers become coated with scale: the cost in heat-transfer efficiency can have a dramatic effect on your power bill! Furthermore, scale can accumulate on the inside of appliances, such as dishwashers, and pipes. As scale builds up, water flow is impeded, and hence appliance parts and pipes must be replaced more often than if Ca2+ and Mg2+ ions were not present in the water.
