Chain growth polymerization:
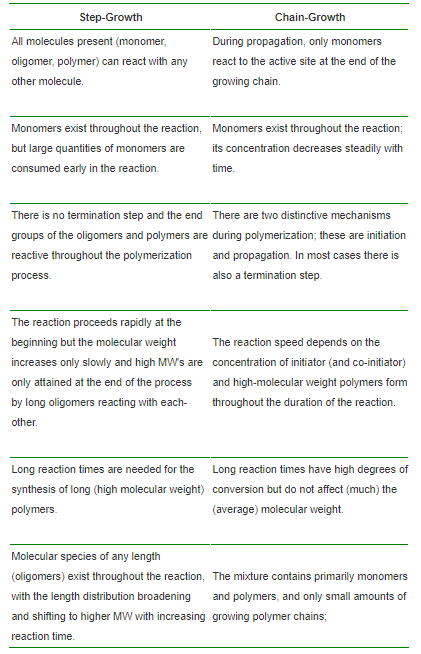
Chain growth polymerization is a polymerization technique where unsaturated monomer molecules add onto the active site on a growing polymer chain one at a time.There are a limited number of these active sites at any moment during the polymerization which gives this method its key characteristics.
Chain growth polymerization has the following characteristics
- growth of polymer occurs by adding monomers to relatively few polymer chains;
- monomer remains even at long reaction times;
- average molar mass increases quickly;
- initiation is required.
Step-growth polymerization:
A step-growth polymerization is a stepwise reaction between bi-functional or multi-functional monomers in which high-molecular-weight polymers are formed after a large number of steps. In contrast to chain-growth, all monomers are reactive. As a consequence, most monomers are consumed early in the polymerization to form short chains (oligomers) that combine to long polymer chains at a later stage of the polymerization.
Many naturally and synthetic polymers are produced by step-growth polymerization including polyesters, polyether, urethanes, epoxies, and polyamides.Two well-known examples are the reaction of dicarboxylic acids with diamines to form polyamides (Nylon) and the reaction of organic diacids with alcohols to form polyesters, like polyethylene terephthalate (PET).
HOOC-R’-COOH + HO-R”-OH → HOOC-R’-COO-R”-OH + H2O
HOOC-R’-COOH + H2N-R”-NH2 → HOOC-R’-CONH-R”-NH2 + H2O
Due to the nature of the polymerization mechanism, the reaction has to proceed for a long time to achieve high molecular weight polymers. The easiest way to visualize a step-growth polymerization mechanism is a crowd of people reaching out to hold their hands to form human chains – each person has two hands (= two reactive sites) and can hold hands (form bonds) with two other persons.
Difference Between Chain and Step Growth Polymerization: