Conductive Polymers:
Conductive polymers or, more precisely, intrinsically conducting polymers (ICPs) are organic polymers that conduct electricity. Such compounds may have metallic conductivity or can be semiconductors. The biggest advantage of conductive polymers is their processability, mainly by dispersion.
Conductive polymers are generally not thermoplastics, i.e., they are not thermoformable. But, like insulating polymers, they are organic materials. They can offer high electrical conductivity but do not show similar mechanical properties to other commercially available polymers. The electrical properties can be fine-tuned using the methods of organic synthesis and by advanced dispersion techniques.
Conducting polymers have backbones of continuous sp2 hybridized carbon centers. One valence electron on each center resides in a pz orbital, which is orthogonal to the other three sigma-bonds. The electrons in these delocalized orbitals have high mobility.
Classification of Conducting Polymers:
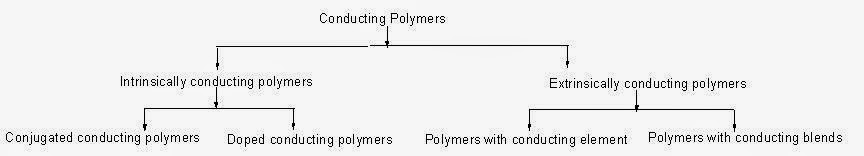
The conduction of electricity in this type of polymers is due to conjugation in the backbone of the polymer. The conjugation can be due to either pi electrons or due to doped ingredients.
Conduction due to Conjugated Pi-Electrons:
In these types of polymers, due to the presence of double bonds and lone pair of electrons conduction of electricity takes place.
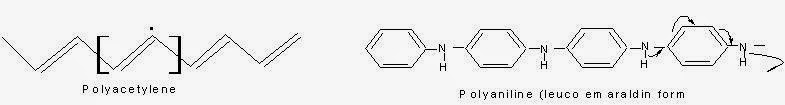
Actually due to overlapping of conjugated pi-electrons, there develops valance and conduction bands through out the backbone of the polymer. Electrical conduction can occur only after attainment of required energy of activation either thermally or photo chemically (because there will be some gap between the valance and conduction bonds. So the electrons need to be exited by some means).
Doped Conducting Polymers:
(i). p-Doping:
The polymers which have conjugation in the back bone when treated with electron – deficient species (Lewis acids) like FeCl3 or I2 vapour or I2/CCl4 ,there takes place oxidation and a positive charge is created in the molecule. Removal of one electron from the pi-back bone of a conjugated polymer forms a radical cation (polaron), which on losing another electron forms bipolaron. The delocalization of positive charges causes electrical conduction.
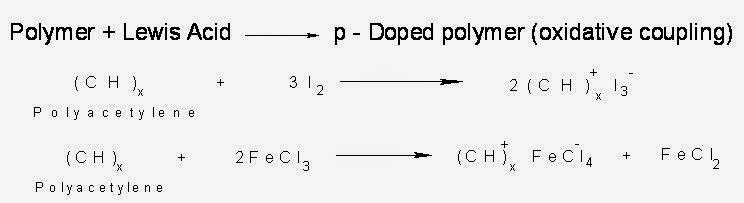
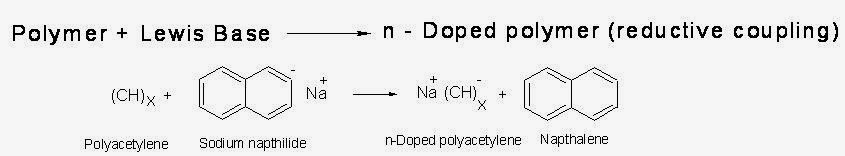
(ii.)n-doping:
When Lewis bases (electron rich species) are treated with polymers having conjugation, due to reduction of the polymer, negative charge develops. Actually by the addition one electron, polaron and by the addition of second electron, bipolaron are formed. In bipolaron, due to the delocalization of the charge, conduction takes place.
Intrinsically conducting polymers are characterized by good electrical conductivity, capability to store charge, capacity to exchange ions, ability to absorb visible radiation thereby yielding the coloured compounds. These are also X-ray transparent.
Extrinsically Conducting Polymers:
The conductivity of these polymers is due to the addition of external ingredients.
When carbon black or some metal oxides or metallic fibers are added, the polymer becomes conductive. The minimum concentration of the element required for the conductivity is called percolation threshold. The filler (ingredients) that percolate have more surface area, more porosity and filamentous nature due to which they can enhance the conducting properties.
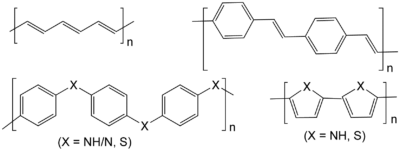
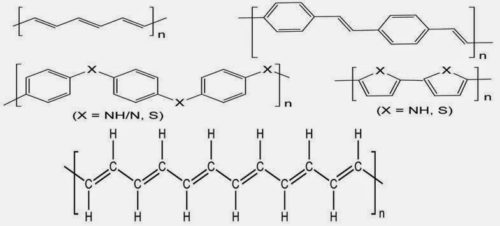
Polyphenylenevinylene, polyacetylene, polythiophene (X = S)
and polypyrrole (X = NH), polyaniline (X = N, NH) and polyphenylene sulfide (X=S).
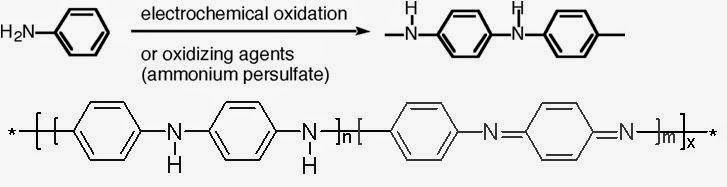
Poly Aniline:
It an be synthesized by the oxidative polymerization of aniline in presence of ammonium persulphate dissolved in 1M HCl. The minimum concentration of the element required for the conductivity is called percolation threshold.
The filler (ingredients) that percolate have more surface area, more porosity and filamentous nature due to which they can enhance the conducting properties.
Polyphenylenevinylene, polyacetylene, polythiophene (X = S)
and polypyrrole (X = NH), polyaniline (X = N, NH) and polyphenylene sulfide (X=S).

Hey ..it’s nice
ok
As in the case of semiconductors, extrinsic semiconductors are those which are doped by some pentavalent or divalent impurity, and intrinsic semiconductors have no impurity (doped atoms). My question is, why do we categorize doped conducting polymers as “intrinsically conducting polymers”? Why “doped conducting polymers” are not “extrinsically conducting polymers”?