The hardness of water:
Many industrial and domestic water users are concerned about the hardness of their water. Hard water requires more soap and synthetic detergents for home laundry and washing, and contributes to scaling in boilers and industrial equipment. Hardness is caused by compounds of calcium and magnesium, and by a variety of other metals. Water is an excellent solvent and readily dissolves minerals it comes in contact with. As water moves through soil and rock, it dissolves very small amounts of minerals and holds them in solution. Calcium and magnesium dissolved in water are the two most common minerals that make water “hard.”
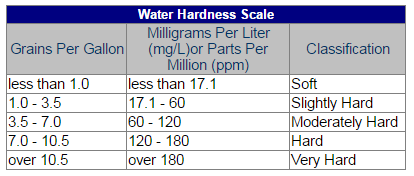
The hardness of water is referred to by three types of measurements: grains per gallon, milligrams per liter (mg/L), or parts per million (ppm). Typically, the water produced by Fairfax Water is considered “moderately hard” to “hard.” The table below is provided as a reference.
Types of Hardness of Water:
The hardness of water can be either
- temporary or
- permanent
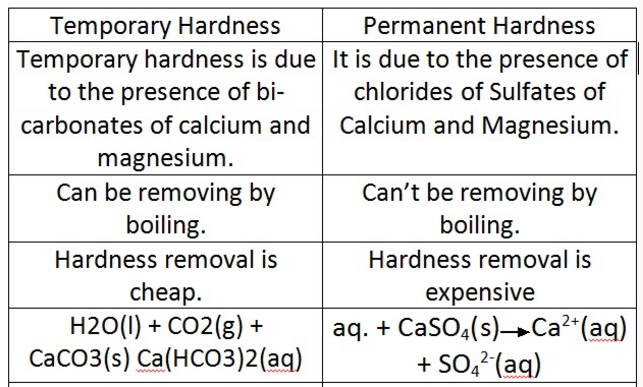
Temporary hardness can be removed simply by boiling the water .
Permanent hardness cannot be removed by boiling but can often be removed by chemical treatment .
Temporary Hardness of Water:
Temporary hardness is caused by dissolved calcium hydrogencarbonate, Ca(HCO3)2.
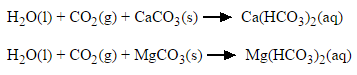
Rainwater is naturally acidic because it contains dissolved carbon dioxide from the air. It reacts with calcium carbonate in rocks to form calcium hydrogencarbonate (which is soluble):
calcium carbonate + water + carbon dioxide → calcium hydrogencarbonate
Temporary hardness is removed by boiling the water. When this happens, the soluble calcium hydrogencarbonate decomposes (breaks down) to form calcium carbonate (which is insoluble), water and carbon dioxide:
calcium hydrogencarbonate → calcium carbonate + water + carbon dioxide
Ca(HCO3)2 → CaCO3 + H2O + CO2
The insoluble calcium carbonate forms a layer of limescale. This may coat the heating element in kettles and irons, for example, making it less efficient. Limescale is unsightly and it clogs up hot water pipes and boilers .
Permanent Hardness of Water:
Permanent hardness is caused by dissolved calcium sulfate. Unlike temporary hardness, it is not removed by boiling the water.
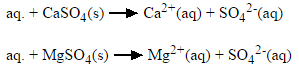
When this is the case, it is usually caused by the presence of calcium sulphate/calcium chloride and/or magnesium sulphate/magnesium chloride in the water, which do not precipitate out as the temperature increases. Ions causing permanent hardness of water can be removed using a water softener, or ion exchange column.
Total Permanent Hardness = Calcium Hardness + Magnesium Hardness
The calcium and magnesium hardness is the concentration of calcium and magnesium ions expressed as equivalent of calcium carbonate.
Difference Between Permanent and Temporary Hardness: