Introduction:
In solids, electrons in outer most orbits of atoms determine its electrical properties. Electron theory is applicable to all solids, both metals and non metals. In addition, it explains the electrical, thermal and magnetic properties of solids. The structure and properties of solids are explained employing their electronic structure by the electron theory of solids. It has been developed in three main stages.
1. Classical free electron theory
2. Quantum Free Electron Theory
3. Zone Theory
1. Classical free electron theory: The first theory was developed by Drude and Lorentz in 1900. According to this theory, metal contains free electrons which are responsible for the electrical conductivity and electrons obey the laws of classical mechanics.
2. Quantum Free Electron Theory: In 1928 Sommerfeld developed the quantum free electron theory. According to Sommerfeld, the free electrons move with a constant potential. This theory obeys quantum laws.
3. Zone Theory: Bloch introduced the band theory in 1928. According to this theory, free electrons move in a periodic potential provided by the lattice. This theory is also called “Band Theory of Solids”. It gives complete informational study of electrons.
1) What are the assumptions of classical free electron theory?
Classical free electron theory of metals (Drude – Lorentz theory of metals): Drude and Lorentz proposed this theory in 1900. According to this theory, the metals containing the free electrons obey the laws of classical mechanics.
Assumptions (or) Salient features in classical free electron theory
1. In metals there are a large number of free electrons moving freely in all possible
directions.
2. These free electrons behave like gas molecules in a container obeying the laws of
kinetic theory of gases.
3.
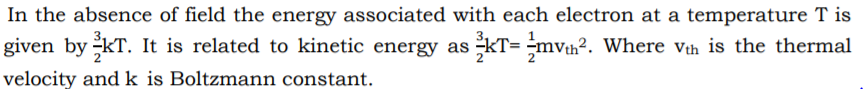
4. In metals, the positive ion cores are at fixed positions and the free electrons move
randomly and collide either with positive ion cores or with other free electrons or with
boundaries. Hence these collisions are elastic. Therefore the electric conduction is due
to free electrons only.
5. Electron velocities in a metal obey Maxwell-Boltzmann distribution of velocities.
6. The free electrons move in a constant potential field. Hence the potential energy of the
electrons is constant.
7. When an electric field is applied to a metal, free electrons are accelerated in the
direction opposite to the direction of applied electric field with a velocity called drift
velocity represented as vd.
2. What are the merits and demerits of classical free electron theory?
Success or Advantages or Merits classical free electron theory
1) It verifies ohm’s law.
2) It explains electrical and thermal conductivities of metals.
3) It derives Widemann-Franz law.
4) It explains optical properties of metals.
Limitations or drawbacks or Demerits classical free electron theory
1) It fails to explain the electrical conductivity of semiconductors and insulators.
2) It fails to explain the temperature variation of electrical conductivity at low
temperature.
3) It fails to explain the concept of specific heat of metals.
4) It fails to explain the mean free path of the electrons.
5) The phenomenon like photo electric effect, Compton effect and black body radiation
could not be explained by classical free electron theory.
6) It fails to explain temperature dependence of paramagnetic susceptibility and
ferromagnetism.
3) What are the assumptions of quantum free electron theory?
Quantum Free Electron Theory:
Quantum free electron theory was proposed by Sommerfeld in 1928. It overcomes many
of the drawbacks of classical theory. Sommerfeld explained them by choosing FermiDirac statistics instead of Maxwell-Boltzmann statistics. He developed this theory by
applying the principles of quantum mechanics.
Assumptions of Quantum Free Electron Theory
1) Valence electrons move freely in a constant potential within the boundaries of metal
and is prevented from escaping the metal at the boundaries (high potential). Hence the
electron is trapped in a potential well.
2) The distribution of electrons in various allowed energy levels occurs as per Pauli
Exclusion Principle.
3) The attraction between the free electrons and lattice ions and the repulsion between
electrons themselves are ignored.
4) The distribution of energy among the free electrons is according to Fermi-Dirac
statistics.
5) The energy values of free electrons are quantized.
6) To find the possible energy values of electron Schrodinger time independent wave
equation is applied. The problem is similar to that of particle present in a potential box.
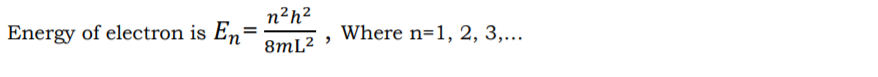
4. What are the merits and demerits of quantum free electron theory?
Merits of quantum free electron theory
1. It successfully explains the electrical and thermal conductivity of metals.
2. It can explain the Thermionic phenomenon.
3. It explains temperature dependence of conductivity of metals.
4. It can explain the specific heat of metals.
5. It explains magnetic susceptibility of metals.
6. It can explain photo electric effect, Compton Effect and block body radiation etc.
7. It gives the correct mathematical expression for the thermal conductivity of metals.
Demerits of quantum free electron theory
1. It is unable to explain the metallic properties exhibited by only certain crystals.
2. It is unable to explain why the atomic arrays in metallic crystals should prefer certain
structures only.
3. This theory fails to distinguish between metal, semiconductor and Insulator.
4. It also fails to explain the positive value of Hall Co-efficient.
5. According to this theory, only two electrons are present in the Fermi level and they
are responsible for conduction which is not true.

A free electron when once snaps it’s connection with the parent atom how can pauli exclusion principle be applied to the
free atom. Could you please explain? Thank you in advance.
Is the energy of electrons in electron gas quantized? If not is it necessary to obey PEP?