Lime-Soda Water Softening Process:
Soda lime is a process used in water treatment to remove Hardness from water. This process is now obsolete but was very useful for the treatment of large volumes of hard water. Addition of lime (CaO) and soda (Na2CO3) to the hard water precipitates calcium as the carbonate, and magnesium as its hydroxide. The amounts of the two chemicals required are easily calculated from the analysis of the water and stoichiometry of the reactions. The lime‐soda uses lime, Ca(OH)2 and soda ash, Na2CO3, to precipitate hardness from solution.
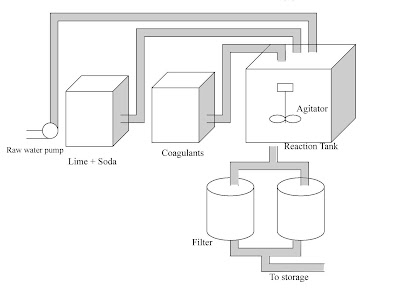
Carbon dioxide and carbonate hardness (calcium and Magnesium bicarbonate) are complexed by lime. In this process Calcium and Magnesium ions are precipitated by the addition of lime (Ca(OH)2) and soda ash (Na2CO3).
Chemistry of Precipitation Softening:
In almost every raw water supply, hardness is present as calcium and magnesium bicarbonate, often referred to as carbonate hardness or temporary hardness. These compounds result from the action of acidic, carbon dioxide laden rain water on naturally occurring minerals in the earth, such as limestone. For example:
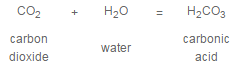

Hardness may also be present as a sulfate or chloride salt, referred to as noncarbonate or permanent hardness. These salts are caused by mineral acids present in rain water or the solution of naturally occurring acidic minerals.
The significance of “carbonate” or “temporary” hardness as contrasted to “noncarbonate” or “permanent” hardness is that the former may be reduced in concentration simply by heating. In effect, heating reverses the solution reaction:
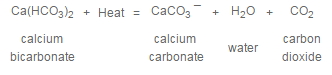
Reduction of noncarbonate hardness, by contrast, requires chemical addition. A combination of lime and soda ash, along with coagulant and flocculant chemicals, is added to raw water to promote a precipitation reaction. This allows softening to take place.
Cold Lime Softening Process:
Precipitation softening accomplished at ambient temperatures is referred to as cold lime softening. When hydrated lime, Ca(OH)2, is added to the water being treated, the following reactions occur:
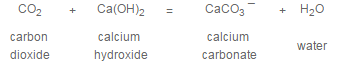
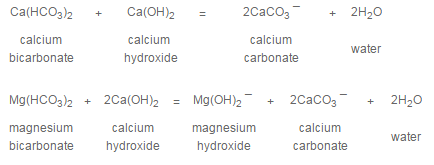
Noncarbonate or permanent calcium hardness, if present, is not affected by treatment with lime alone. If noncarbonate magnesium hardness is present in an amount greater than 70 ppm and an excess hydroxyl alkalinity of about 5 ppm is maintained, the magnesium will be reduced to about 70 ppm, but the calcium will increase in proportion to the magnesium reduction.
If the proper chemical control is maintained on lime feed, the calcium hardness may be reduced to 35-50 ppm. Magnesium reduction is a function of the amount of hydroxyl (OH–) alkalinity excess maintained. Figures 7-1 and 7-2 show these relationships.
Warm Lime Softening Process:
The warm lime softening process operates in the temperature range of 120-140°F (49-60°C). The solubilities of calcium, magnesium, and silica are reduced by increased temperature. Therefore, they are more effectively removed by warm lime softening than by cold lime softening. This process is used for the following purposes:
- To recover waste heat as an energy conservation measure. The water to be treated is heated by a waste stream, such as boiler blowdown or low-pressure exhaust steam, to recover the heat content.
- To prepare feed to a demineralization system. The lower levels of calcium, magnesium, and especially silica reduce the ionic loading on the demineralizer when warm lime-softened water is used rather than cold lime-softened water. This may reduce both the capital and operating costs of the demineralizer. However, most strong base anion resins have a temperature limitation of 140°F (60°C); therefore, additional increases in temperature are not acceptable for increasing the effectiveness of contaminant reduction.
- To lower the blowdown discharge from cooling systems. Cooling tower blowdown may be treated with lime and soda ash or caustic to reduce calcium and magnesium levels so that much of the blowdown may be returned to the cooling system. Silica levels in the recirculating cooling water are also controlled in this manner.
In any warm lime or warm lime-soda ash process, temperature control is critical because temperature variations of as little as 4°F/hr (2°C/hr) can cause gross carryover of the softener pricipitates.
Hot Process Softening Process:
Hot process softening is usually carried out under pressure at temperatures of 227-240°F (108-116°C). At the operating temperature, hot process softening reactions go essentially to completion. This treatment method involves the same reactions described above, except that raw water CO2 is vented and does not participate in the lime reaction. The use of lime and soda ash permits hardness reduction down to 0.5 gr/gal, or about 8 ppm, as calcium carbonate.
Magnesium is reduced to 2-5 ppm because of the lower solubility of magnesium hydroxide at the elevated temperatures.
Advantages of Lime-Soda Process :
- it is very economical.
- if this process is combined with sedimentation with coagulation, lesser amounts of coagulants shall be needed.
- The process increases the pH value of the treated-water; thereby corrosion of the distribution pipes is reduced.
- Besides the removal of hardness, the quantity of minerals in the water is reduced.
- To certain extent, iron and manganese are also removed from the water.
- Due to alkaline nature of treated-water, amount of pathogenic bacteria in water is considerably reduced.
Disadvantage of Lime-Soda Process:
- For efficient and economical softening, careful operation and skilled supervision in required.
- Disposal of large amounts of sludge (insoluble precipitate) poses a problem. However, the sludge may be disposed off in raising low-lying areas of the city.
- This can remove hardness only up to 15 ppm, which is not good for boilers.

Good notes