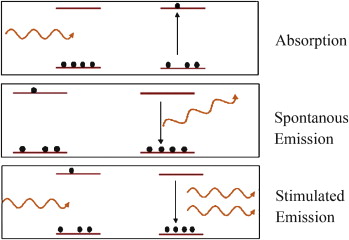
When an atom jumps from a higher energy stated to a lower energy state it emits light in the form of photons. In any source of light, the light that is emitted is incoherent, i.e., different photons have different phases and different wavelengths. The direction of light emitted is also not the same. Such a type of emission happening spontaneously is called spontaneous radiation.
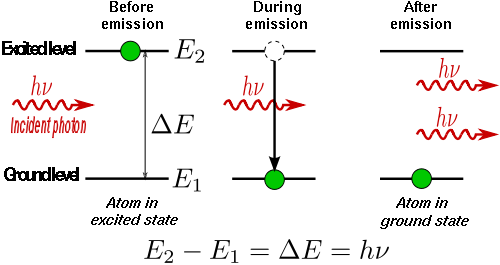
If an atom is in an excited state, it may spontaneously decay into a lower energy level after some time, releasing energy in the form of a photon, which is emitted in a random direction. This process is called spontaneous emission.
