What is Graphite?
Graphite is a crystalline form of the element carbon with its atoms arranged in a hexagonal structure. It occurs naturally in this form and is the most stable form of carbon under standard conditions. Graphite has high thermal and electrical conductivity and high thermal stability. Mainly at temperatures of 700 °C and above, the crystal carbon undergoes oxidation to form CO2.
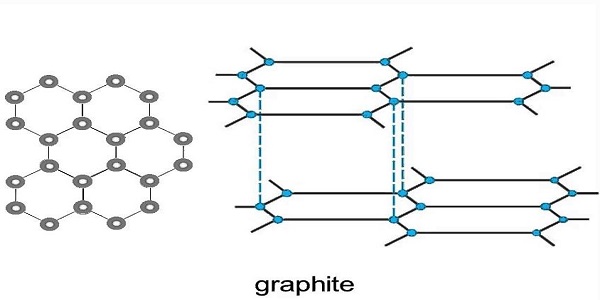
Structure:
- This crystal carbon has a structure that is planar and layered. Graphene is the term used to denote each layer of the same.
- Every layer has atoms of carbon arranged in a honeycomb-like network
- There is a covalent bonding for atoms in the plane with the criteria being met by only three out of four probable bonding sites.
- The graphite would be conducive electrically owing to the fourth electron having a chance to migrate into the plane.
- The layers of the carbon crystal could swiftly move past each other as the layers could be separated easily as van der Waals bonds that are weak-hold them together.
Applications of Graphite:
- In modern world, Graphite is usually used in processes like steelmaking, brake linings, lubricants, foundry facings, batteries etc.
- The uses of the crystal include electrodes and refractories used in applications for processing materials in high temperature.
