What is zeolite?
Zeolite is micro-porous mineral which is used as catalyst in many industrial purposes such as water purification and air purification. The zeolites are hydrated aluminosilicates and general composition AlxSiyO2(x+y) (without water molecules). Zeolites are two types natural and synthetic or artificial.
The natural zeolite that is used for water softening is gluconites or greensand. Permutit is the synthetic zeolite that is most used in water softening and its chemical formula is Na2O, Al2O3, nSiO2, xH2O. These are used as ion exchanger and odor removal in water softener. Permutit are more porous, glassy, and have higher softening capacity than greensand.
Zeolites are characteristically soft to moderately hard, light in density, insoluble in water but can act as base exchangers in contact with water containing cations. Hence these can remove Ca2+ and Mg2+ ions from water when hard water is passes through.
Zeolite Process for Water Softening:
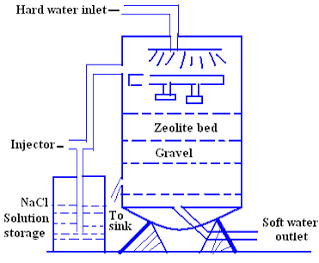
Zeolite process for water softening has become a commercial success for the reason that zeolite can be easily regenerated. When Ca2+ and Mg2+ ions containing hard water is passes through a bed of sodium zeolite, the sodium ions are replace by the calcium and magnesium ions.
Na2Ze + Ca(HCO3)2 → 2NaHCO3 + CaZe
Na2Ze + Mg(HCO3)2 → 2NaHCO3 + MgZe
Na2Ze + CaSO4 → Na2SO4 + CaZe
Na2Ze + MgSO4 → Na2SO4 + MgZe
When all sodium ions are replaced by calcium and magnesium ions, the zeolite becomes inactive. Then the zeolite needs to be regenerated. Brine solutions are passing through the bed of inactivated zeolite. The following reactions are taken place and form Na2Ze.
CaZe + 2NaCl → Na2Ze + CaCl2
MgZe + 2NaCl → Na2Ze + MgCl2
The softening water by this process is used for laundry process and cannot be used for boiler purpose. Because this water softening system contains NaHCO3 in the water; when this water is heated, it produces CO2 which is corrosive for boilerplates.
Limitation of Zeolite Process:
- If the supply of water is turbid, the suspended matte must be removed (by coagulation, filtration, etc.), before the water is admitted to the zeolite bed; otherwise the turbidity will clog the pores of zeolite bed, thereby making it inactive.
- If water contains large quantities of coloured ions such as Mn2+ and Fe2+, they must be removed first, because these ions produce manganese and iron zeolites, which cannot be easily regenerated.
- Mineral acids, if present in water, destroy the zeolite bed and, therefore, they must be neutralized with soda, before admitting the water to the zeolite softening plant.
Advantages of Zeolite Process:
- it removes the hardness almost completely and water of about l0 ppm hardness is produced.
- The equipment used is compact, occupying a small space.
- No impurities are precipitated, so there is no danger of sludge formation in the treated-water at a later stage.
- The process automatically adjusts itself for variation in hardness of incoming water.
- it is quite clean.
- it requires less time for softening.
- it requires less skill for maintenance as well as operation.
Disadvantages of Zeolite Process:
- The treated-water contains more sodium salts than in lime-soda process.
- The method only replaces Ca2+ and Mg2+ ions by Na+ ions, but leaves all the acidic ions like HCO3– and CO32- as such in the softened water. When such softened water (containing NaHCO3, Na2CO3 etc.) is used in boilers for steam generation, sodium bicarbonate decomposes producing CO2, which causes corrosion ; and sodium carbonate hydrolysis to sodium hydroxide, which causes caustic embrittlement.
- High turbidity water cannot be treated efficiently by this method, because fine impurities get deposited on the zeolite bed, thereby creating problem for its working.

Very use full theory
Really, It is very useful theory.