What are Dielectrics?
Dielectrics, in general, can be described as materials that are very poor conductors of electric current. They are basically insulators and contain no free electron. Dielectrics can be easily polarized when an electric field is applied to it. Thus, their behavior in an electric field is entirely different from that of conductors as would be clear from the following discussion.
Dielectric Materials
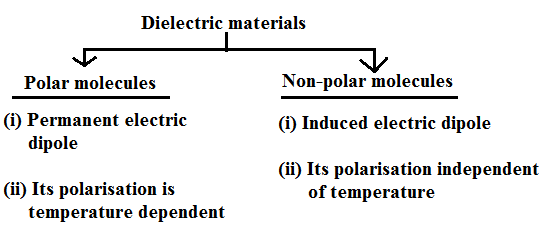
With respect to the atomic view, dielectric materials are classified into two categories:
 Dipolar polarization
Dipolar polarization
Dipolar polarization is a polarization that is either inherent to polar molecules (orientation polarization), or can be induced in any molecule in which the asymmetric distortion of the nuclei is possible (distortion polarization). Orientation polarization results from a permanent dipole, e.g., that arising from the 104.45° angle between the asymmetric bonds between oxygen and hydrogen atoms in the water molecule, which retains polarization in the absence of an external electric field. The assembly of these dipoles forms a macroscopic polarization.
When an external electric field is applied, the distance between charges within each permanent dipole, which is related to chemical bonding, remains constant in orientation polarization; however, the direction of polarization itself rotates. This rotation occurs on a timescale that depends on the torque and surrounding local viscosity of the molecules. Because the rotation is not instantaneous, dipolar polarizations lose the response to electric fields at the highest frequencies. A molecule rotates about 1 radian per picosecond in a fluid, thus this loss occurs at about 1011 Hz (in the microwave region). The delay of the response to the change of the electric field causes friction and heat.
When an external electric field is applied at infrared frequencies or less, the molecules are bent and stretched by the field and the molecular dipole moment changes. The molecular vibration frequency is roughly the inverse of the time it takes for the molecules to bend, and this distortion polarization disappears above the infrared.
